Abstract
Background: Both chronic neutrophilic leukemia (CNL) and atypical chronic myeloid leukemia (aCML) although are rare diseases but pose significant clinical challenge due to poor prognosis. A vast majority of CNL patients harbor mutations in CSF3R that activate both JAK-STAT and MAPK signaling pathways while genetic mutations activating RAS-MAPK pathway (NRAS, SETBP, TET2, ASXL and SRSF2) are seemingly more common in aCML. Recent studies demonstrated that enhanced Mapk signaling is the central feature in both malignancies regardless of driver mutations. Earlier, we have shown that enhanced Mapk signaling fueled by elevated Ksr1 expression is essential for CSF3R induced leukemia. While activation of JAK-STAT signaling was noted with CSF3R mutants, efficacy of Jak2 inhibitor was observed only with CSF3R-proximal mutant (CSF3RT618I). The CSF3R compound mutation (CSF3RT618I/Q740*)which induces more aggressive disease was refractory to ruxolitinib treatment. Interestingly, MEK-ERK signaling by trametinib effectively suppressed the leukemic progression induced by both CSF3R proximal and compound mutations. Recent studies from Maxson and colleagues reported the enhanced Mapk signaling in SETBP induced aCML and response to trametinib treatment in preclinical mouse model. Unfortunately, both trametinib and ruxolitinib exert cytostatic response and are rarely selective to leukemic clones. While treatment with Jak2 or Mek1/2 inhibitors provide some relief to patients, but the responses are short-lived, as leukemic cells in a very short period of time (4-6 months) adapt to thrive under Jak2 and Mek1/2 inhibition resulting to loss of treatment response and disease relapse. In addition, prolonged inhibition of JAK2 and MEK/ERK are seemingly detrimental for normal hematopoietic and adult tissue homeostasis. These observations warrant identifying additional therapeutic targets that must be safe and able to eliminate the mutant clone.
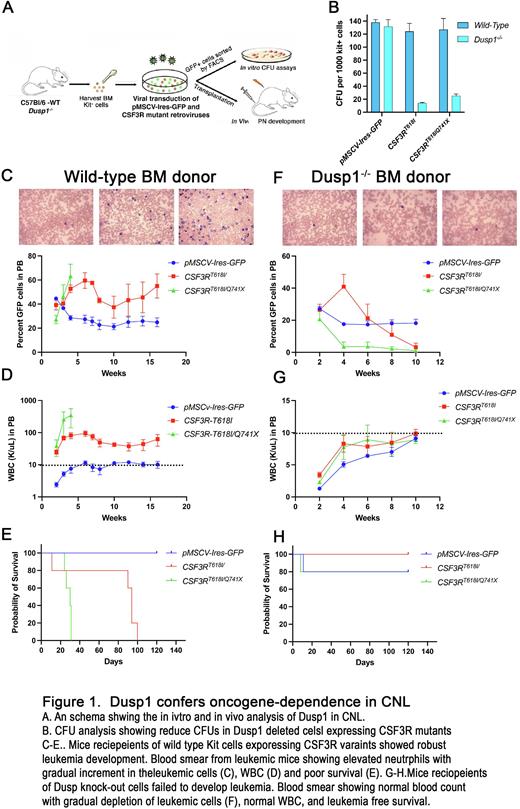
Results: Enhanced and persistent Mapk signaling beside supporting proliferation also strongly stimulate apoptotic signaling which will be counterproductive for cancerous growth. Most tumors exploit the MAPK negative regulators to tame the apoptotic stimuli while maintaining sufficient threshold that can persistently fuel the survival and proliferation. Consequently, cancerous cells induce the expression of MAPK phosphatases (MKPs) to suppress the persistent elevated MAPK signaling induced by oncogenic mutations. Whole genome expression profiling of primary BM cells expressing leukemic and non-leukemic CSF3R revealed induced expression of Dusp1 (Dual specificity phosphatase also called as Mkp1). Genetic deletion of Dusp1 conferred synthetic lethality to leukemic CSF3R variants while sparing normal hematopoiesis (Fig 1). Chemical inhibition of Dusp1 by BCI, which also inhibits Dusp6, failed to leukemic progression. Genetic and biochemical analysis of signaling components revealed that the off-target inhibition of Dusp6 abrogated the anti-leukemic response of BCI by inducing the pERK1/2 mediated survival. Consequently, ectopic expression of Dusp6 or quenching the pERK1/2 signaling by trametinib restored the anti-leukemic response of BCI. Leukemic mice treated with a combination of BCI+trametinib selectively eradicated the leukemic cells resulting to a curative response. Mechanistically, loss of Dusp1 in leukemic context (but not in the normal cells) unleashes the JNK1 driven apoptosis by stabilizing the P53, Bim and degradation of Bcl2.
Conclusion: Our data provide evidence that enhanced Mapk signaling in leukemic cells are regulated by Dusp1 in CNL/aCML. In an analogy to "breaking the break", deletion of Dusp1 resulted in selective eradication of leukemic cells. Altogether, our data supports for developing selective Dusp1 inhibitor for curative treatment outcome.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal